In my quest for battery longevity, I am finding that the temperature that the batteries operate has a substantial bearing on their lifespan.
I think this is generally accepted.
But what is the ideal temperature?
There has been more research on Li-ion batteries than Lithium Iron phosphate. Too low is not good and too high is not good. I have read accounts that say 15 deg C, 20 deg C and 25 deg C being the ideal.
The higher side of those temperatures being better operational and the lower side being better in terms of less degradation. I can’t find definitive research for LiFePo4 batteries that states exactly a specific temperature.
This brings me on to my next practical thought. It would nigh on impossible to keep an active battery bank at a specific temperature. The act of charging and discharging will create heat.
I think it is fair to say that the ambient temperature during the night will be lower than the day though, so there should be some sort of daily temperature swing.
Well is that good enough, I don’t know. It may be perfectly acceptable if you have a set-up that naturally swings between 15 and 25 degrees daily.
Although, when I tracked my lead-acid bank, (admittedly it was in a fresh air environment), it could swing between about 3 and 43 over the year, with a 30-degree daily swing probably being the norm.
I think trying to prevent that swing in an open environment would not have been cost-effective.
Now considering LiFePo4 batteries, I am more comfortable with having those in a closed environment, because of the absence of outgassing. So perhaps temperature control is an option now?
It is easy enough to do electrically. Turn on a cooler when they are too hot, turn on a heater when they are too cold. But that doesn’t float my boat, using electrical power to store energy.
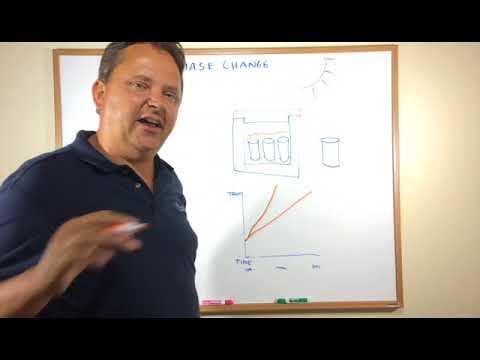
This is where I think “PCM” has a role. “PCM” stands for “phase change materials”.
So what are they when they are at home?
Perhaps an example we all know, water. Water can exist as a solid and a liquid at its melting point (0 deg C). To heat a litre of liquid water 1 deg C takes 4.18kJ/kg.
However, to turn a litre of water at 0 deg C into a litre of ice at 0 deg C takes 333.6 kJ/kg.
In practical terms that means the same amount of energy has been transferred to change the phase of water from liquid to ice as would be needed to heat 1 litre of water from 0 deg C to 80 deg C.
This is also a well-known phenomenon, it is called latent heat and the temperature of the medium does not actually change.
Ok, fair enough, where does this fit in? Well, it means that if I put a 5-litre bottle of water in my freezer. It would slow my freezer down as it tried to reach a temperature lower than 0 degrees. And if I had a power cut that bottle would slow that freezer from rising above 0 degrees. This thermal inertia is disproportionate to its physical mass. It would be a thermal battery.
Ok fine, but I don’t want to keep my Lithium battery bank at 0 degrees. I want to keep it at somewhere between 15 and 25 degrees. Well, PCM’s come in all sorts of signature temperatures. Pick one that freezes/melts at 18 degrees C. The thermal inertia of the PCM would be reluctant to allow the battery enclosure to drop below 18 degrees and likewise, it would be reluctant to allow it to rise above 18 degrees.
In effect a passive heater/cooler ( no moving parts) that would be powered by the natural daily temperature swings.
PS. I can also see applications for this for hot water storage for extended periods.